Lyme disease is usually described as an easily treated infection: A tick bites you. It transmits a bacterium called Borrelia burgdorferi. You get a rash. Maybe a fever. You take antibiotics. No big deal.
But the more that I read about this mysterious disease, the more that story unraveled. Research suggests Borrelia possesses an array of adaptations that allow it to evade immune defenses and survive antibiotics. It can even lie dormant inside your tissues only to reactivate under more favorable conditions, like a sleeper cell.
Perhaps most shocking, some evidence suggests Borrelia can spread without a tick in sight.
My interest in Lyme started with my mom. She’d battled strange, hard-to-diagnose symptoms for most of her adult life: fatigue, joint pain, brain fog, temperature sensitivity, mood swings, never-ending gut problems, and more recently, neurological issues. When she was finally diagnosed with Lyme after decades of trying to resolve her mysterious health issues, the puzzle pieces snapped into place, which was a huge relief.
She’d lived in Ithaca, New York as a kid, a known hotspot for ticks. All of her symptoms match those of systemic Lyme. It was surreal to realize she had been struggling with undiagnosed Lyme all this time: the news instantly reframed many assumptions I had made about who she was.
Not long after, we discovered my little brother had Lyme too. He’s an avid rock-climber, so he does a lot of traveling and outdoor recreation. He must have been bitten somewhere, I thought. But something struck me as odd about this news: he also struggled with many of the same issues as my mom since he was a kid. If the Lyme diagnosis had finally shed light on my mom’s mysterious symptoms, but my brother was only recently bit on a rock-climbing trip, then why did he have so many of the same strange symptoms growing up?
Then my brother’s girlfriend started developing symptoms that were eerily reminiscent of Lyme. She was subsequently confirmed to be Lyme positive, and that didn’t make sense at all. She’s lived her whole life in Grand Junction, Colorado, far from the forests of the Northeast, and far from any known Lyme epicenter. No tick has ever been found to carry Borrelia burgdorferi in Colorado. That’s when something snapped into focus for me.
If not a tick…then what? Can Lyme spread from person to person? A quick google search made me feel silly for asking.
But as I dug into the literature, I began to think that the answer wasn’t so clear. Take congenital transmission. Borrelia has been found in placental tissue, umbilical cords, and fetal brains, yet the possibility of congenital Lyme has went largely unacknowledged in mainstream medicine and public health guidelines.
That has recently started to change, however.
The 2024 edition of Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant —a gold-standard neonatal medical reference—now explicitly includes B. burgdorferi as a pathogen of concern in congenital infection.
And the CDC now states that mother-to-fetus transmission is possible, though it emphasizes its rarity.
These developments suggests that the scientific tide may be turning with respect to non-tick transmission of Lyme.
And if congenital transmission is now on the table, it raises a natural follow-up question: Could Lyme disease, like its cousin syphilis, exploit sexual transmission?
In the face of skepticism from public health authorities, converging lines of evidence have quietly accumulated, challenging the tick-only paradigm.
A Master of Disguise
It’s commonly assumed that Lyme disease is easily treated with a course of antibiotics. And for many patients who catch it early and are correctly diagnosed, that’s true. But that’s a huge qualifier—many people don’t suspect Lyme at all, especially if they don’t recall a tick bite or live outside of high-risk areas. If the diagnosis is delayed, missed, or dismissed, treatment may come too late, or not at all.
But even for those who are treated, a growing body of evidence suggests that Borrelia burgdorferi is not always so easy to eradicate. In fact, B. burgdorferi may be one of the most elusive pathogens modern medicine contends with.
Persistent infection despite antibiotic treatment has been demonstrated in non-human primates. Embers et al. (2012) infected rhesus macaques via tick bite and treated them with standard antibiotic regimens. Months later, multiple methods revealed persistent, metabolically active Borrelia in heart, brain, and joint tissues, despite appropriate therapy.
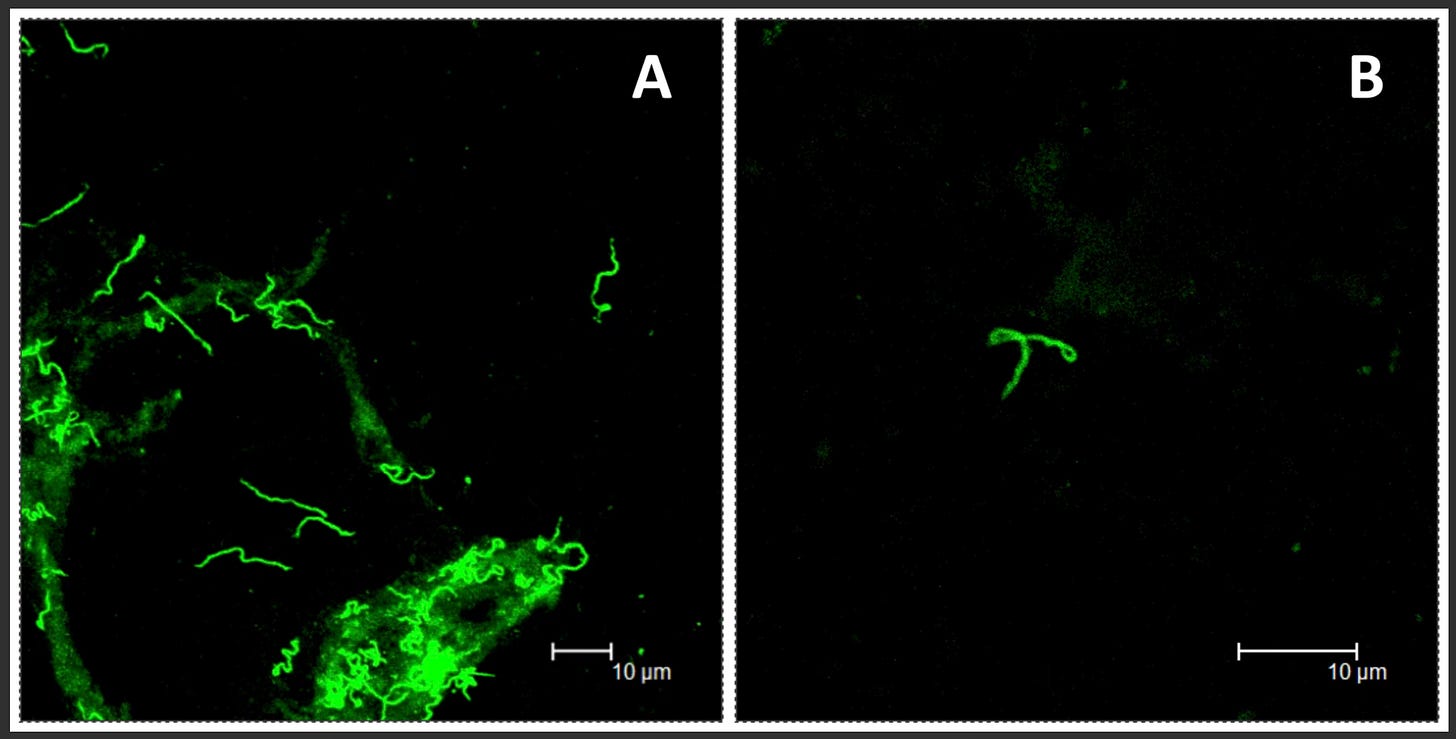
Similar patterns have been observed in humans. Middleveen et al. (2018) identified Borrelia DNA and “atypical forms” of the bacterium in bodily fluids and tissues of patients who continued to experience symptoms after standard antibiotic treatment. The authors concluded that morphological adaptations may play a role in persistent infection and immune evasion.
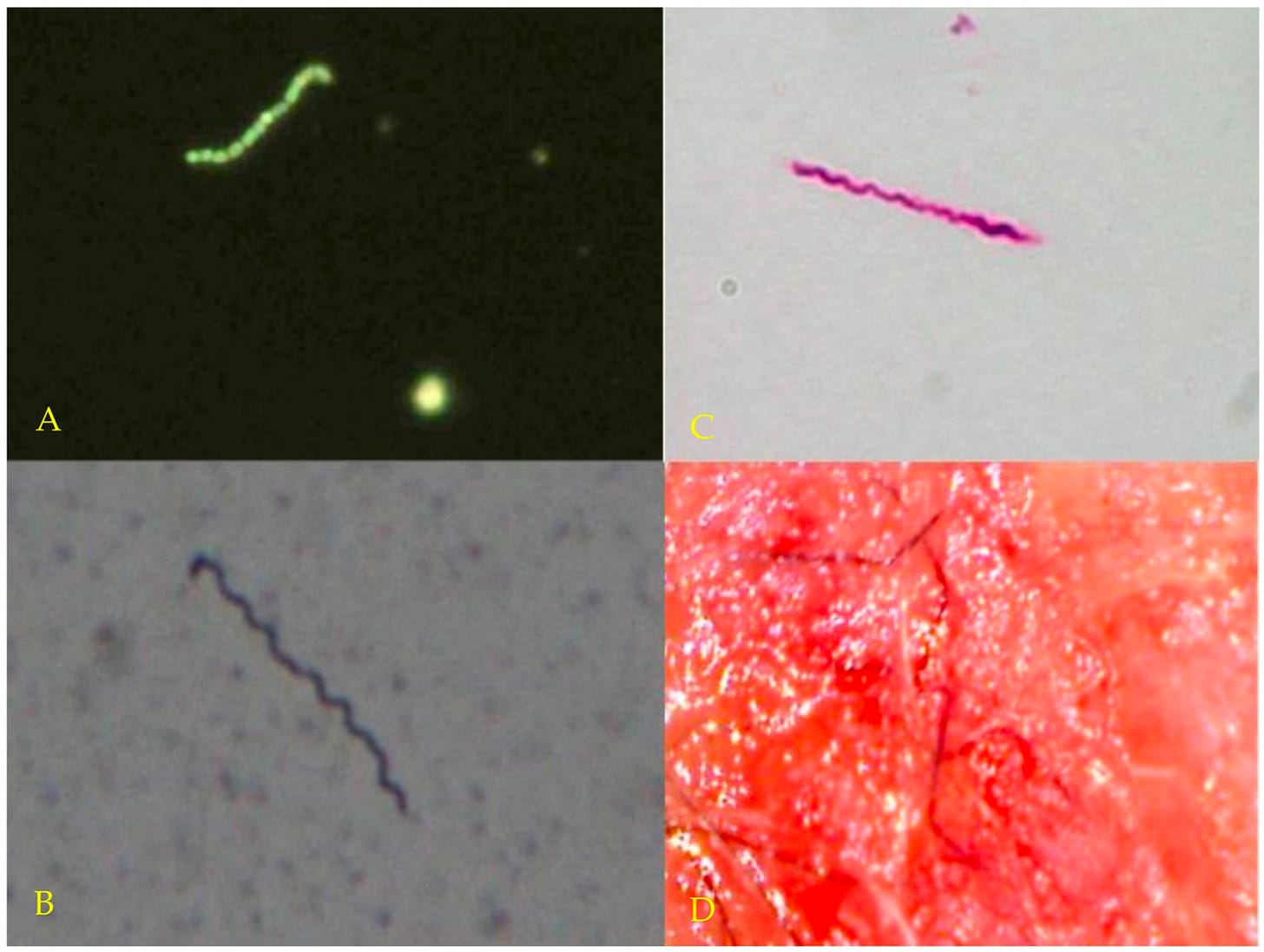
These findings align with decades of research suggesting that Borrelia burgdorferi is a shape-shifter. Its evolutionary edge lies in its ability to switch physical forms, each adapted to a different environment, challenge, or phase of infection.
In its classic spiral shape (spirochetes), Borrelia quickly spreads through viscous environments in the body. Borrelia’s tightly wound helix allows it to drill through connective tissue. It can even enter host cells, setting up shop inside. While intracellular, it’s shielded from antibodies and some antibiotics.
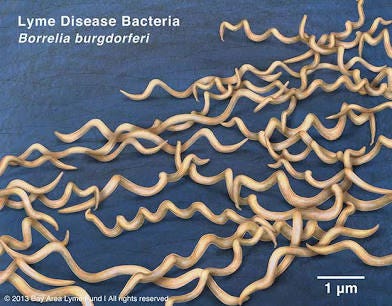
When threatened (by antibiotics, oxidative stress, immune attack), Borrelia retracts into a spherical “cyst” form. In this dormant state, it stops replicating and turns down its metabolism, making itself less visible to immune detection and diagnostic tests that depend on active bacterial markers. These forms are also highly resistant to antibiotics.
It can stay hidden for weeks, months, possibly even years, only to reactivate opportunistically under more favorable conditions, such as when its host is under stress or experiencing immune suppression.
And it doesn’t stop there. In vitro and in vivo evidence suggests Borrelia also employs social coordination to overcome environmental challenges. Once established, Borrelia gathers into communities, forming dense biofilms—slimy protective matrices. Inside these cocoons, Borrelia coordinate their behavior and share genetic material. Biofilms have been identified in synovial fluid, heart valves, skin lesions, and the bladder. Within these micro-communities, Borrelia becomes less susceptible to antibiotic treatment.
All the while, Borrelia alters its surface proteins, effectively changing its disguise to stay one step ahead of the immune system. It even deploys complement resistance proteins that interfere with the host’s innate bacterial kill switch.
If Borrelia isn’t where we expect it, and isn’t expressing the markers we test for, we may not see it at all. And that’s honestly kind of scary.
A Diagnostic Mess
Indeed, Lyme is absurdly hard to diagnose. Its symptoms are vague, multifaceted, and often overlap with many other conditions: It can look like depression, fibromyalgia, chronic fatigue, multiple sclerosis, ALS, even aging. This ambiguity makes it easy to miss or misattribute, especially in the absence of a known tick bite. The musician Kris Kristofferson, for instance, was told he had Alzheimer’s, until doctors later realized it was Lyme.
And the standard tests don’t make things much clearer. The typical two-tier process (ELISA followed by Western blot) detects Borrelia-specific antibodies, so it can’t distinguish between past and present infections, nor can it confirm whether the infection has been successfully cleared.
Even more limiting, the standard Western blot test used in the U.S. is designed to detect only Borrelia burgdorferi sensu stricto. Yet dozens of Borrelia strains—many of them falling under the broader B. burgdorferi sensu lato complex—circulate across the U.S., and some may be better at evading detection than others. Common co-infections, including Babesia, Bartonella, and Ehrlichia, are typically not tested for unless patients pay out-of-pocket for specialized labs. To this point, a survey of over 3,000 chronic Lyme patients found that over half had at least one co-infection. Yet standard testing rarely screens for these, and accessing the appropriate diagnostics typically requires out-of-pocket costs and specialist referrals.
In addition, Borrelia may not trigger a detectable antibody response for weeks after infection. During this window, tests can return negative even when the infection is present and spreading. Standard serological tests can miss up to 35–50% of early Lyme cases, with overall sensitivity for the two-tiered approach estimated at 77%. That’s a 1-in-4 chance of being told you’re fine when you’re not, assuming you even get tested in the first place.
And as we’ve already seen, Borrelia is a shape-shifter, capable of hiding inside cells, forming biofilms, going dormant, and changing its surface proteins to fool the immune system. Which raises the question:
How good is a test for the body’s response if the body struggles to see the intruder?
As a result, underdiagnosis of Lyme is a major issue, and its true prevalence is hard to estimate. That makes it difficult to apply a Bayesian logic with any confidence. A flawed baseline (e.g., assuming Lyme is unlikely) will skew interpretation of both positive and negative tests. Many patients (like my mom) can go undiagnosed for years.
In fact, an analysis of 100 Lyme disease patients with neuropsychiatric symptoms showed that the average time from infection to diagnosis was about 9 years. That’s not a typo—nine years.
Even when Lyme is suspected, this diagnostic gap leaves both patients and clinicians in the dark: persistent symptoms may reflect ongoing infection, lingering immune dysfunction, or something else entirely. And the reverse is also true: A reduction in symptoms does not necessarily confirm that the infection has been cleared. Borrelia can adopt various forms that suppress immune detection. Symptoms may therefore ease while the infection persists in hiding. Without a standardized testing protocol that can reliably detect active Borrelia in the body—or rule it out—those questions remain unresolved.
Is the infection gone? Is it hiding? Is the immune system still reacting to something that’s no longer there?
We often don’t know.
Parallels to Syphilis
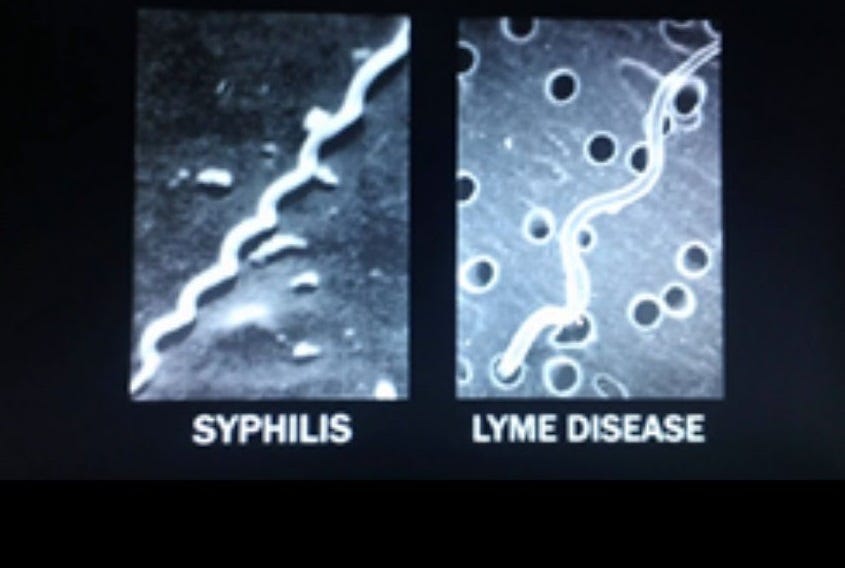
Syphilis, caused by the spirochete Treponema pallidum, was once thought to be a simple, local infection. An uncomfortable sore, easily treated. But that story didn’t hold. Over time, syphilis revealed itself as a sexually transmitted pathogen that could go quiet for years, slip into the nervous system, cross the placenta into unborn children, and return decades later as madness or paralysis. It earned its grim nickname “the great imitator” for good reason.
Borrelia behaves similarly, demonstrating that is shares more than just a family tree with T. pallidum. It, too, can enter the brain. It, too, can hide in immune-privileged corners of the body. It, too, has been found in semen, vaginal fluid, and fetal tissue. Like syphilis, Borrelia causes a wide spectrum of symptoms across multiple systems—musculoskeletal, neurological, dermatological, and psychiatric—and is notoriously difficult to diagnose.
Both spirochetes even release tiny packages of surface proteins and DNA that trick the immune system into chasing decoys while the real bacteria evade capture.
These phenotypic parallels set a precedent. If one spirochete evolved the ability to transmit sexually, is it really so implausible that its cousin could do the same, given their shared ancestry and morphological similarities?
Although Borrelia is adapted to tick-borne transmission, it’s not unusual for pathogens to evolve multiple routes of spread, especially if those routes enable persistence in the host population. Indeed, Zika and Ebola, both primarily vector- or contact-transmitted, have shown evidence of sexual transmission.
Animal Models
If Lyme can spread without a tick, then where’s the evidence?
One place to look is in animal models, especially the small mammals that serve as natural reservoirs for Borrelia. And indeed, some of the earliest clues came from lab mice.
In 1986, Burgess and colleagues reported that uninfected wild mice (Peromyscus leucopus and P. maniculatus) housed with infected mice became infected through direct contact. No ticks, no bites, just cohabitation. All of the exposed mice developed antibodies to Borrelia burgdorferi, and one even had live spirochetes cultured from its blood. The researchers concluded that transmission had occurred “without an arthropod vector.” A follow-up study in 1990 by Wright and Nielsen confirmed that direct contact could transmit Lyme infection between mice.
Altaie et al. (1997) added an intriguing wrinkle. In this study, infected male mice were mated with uninfected females, and the resulting fetuses and placentas were tested. Borrelia DNA was detected in a subset of samples—13% of fetuses and 10% of placentas—especially during early pregnancy. In a second phase of the study, researchers let pregnancies proceed to term and checked the pups. Some tested positive for Borrelia within 24 hours of birth, others two weeks later. Intriguingly, nine infected pups came from pairings where only the male was infected. That hints at possible venereal transmission from sire to dam, and then vertical transmission to the fetus.
But not all animal studies tell the same story.
In a 1991 study, Moody and Barthold tested transmission in rats by cohabitating infected and uninfected rats for 30 days. None of the uninfected rats acquired Lyme infection, despite prolonged intimate contact with infected cage-mates. They also specifically attempted to breed infected rats with uninfected rats, but no venereal transmission occurred from either infected females or infected males in the controlled rat experiments.
A 1999 study by Woodrum and Oliver in Syrian hamsters also found no evidence of Lyme spreading via mating. The researchers paired six infected female hamsters with uninfected males and three infected male hamsters with uninfected females; not a single uninfected partner became infected after mating. Nor did they find evidence of transmission through casual contact or urine/feces.
Why the inconsistency?
One possibility is species differences. Mice—especially Peromyscus—are natural Borrelia hosts, and may harbor spirochetes in mucosal surfaces more readily. Hamsters and rats, on the other hand, may not support the same level of colonization, or may not shed infectious forms in semen or vaginal secretions at sufficient density to cause infection. That doesn’t mean sexual transmission can’t happen, only that it may not happen in those species under the studied conditions.
Methodology also matters. Some researchers have noted that the negative studies used relatively short contact times and relied solely on serology to assess transmission. That means they may have missed low-level or localized infections, especially if animals didn’t mount a strong antibody response.
Indeed, a few missed signals doesn’t mean the signal isn’t there.
In the early 1990s, a veterinarian researcher, John Gustafson, investigated Lyme transmission in canines. In his 1993 Ph.D. thesis, Gustafson provided compelling evidence that Borrelia burgdorferi can be transmitted from mother to offspring in dogs. Ten female beagles were experimentally infected with Borrelia during early pregnancy and then bred naturally. Eight of the ten infected females delivered litters in which at least one pup tested positive for Borrelia DNA, and viable spirochetes were successfully cultured from the tissues of pups in two of the litters. Gustafson’s experiment study was ultimately published in the American Journal of Veterinary Research. This study didn’t test for sexual transmission—but it did confirm something equally important: that Borrelia can cross the placenta and infect offspring in utero.
Taken together, these results are messy. But when you step back, a pattern emerges: In some hosts, Borrelia burgdorferi may be more mobile than we thought, capable of exploiting direct contact, mucosal exposure, and reproductive pathways to spread. That doesn’t mean non-tick transmission is common or efficient. But it does mean it deserves a closer look.
Sexual Transmission in Ticks
Borrelia’s ability to transmit sexually is well documented…in its tick vectors.
Back in the 1950s and 1980s, researchers observed venereal transmission of relapsing fever Borrelia species (like B. duttoni and B. crocidurae) in soft ticks. During copulation, infected male ticks passed the spirochetes directly to their female partners.
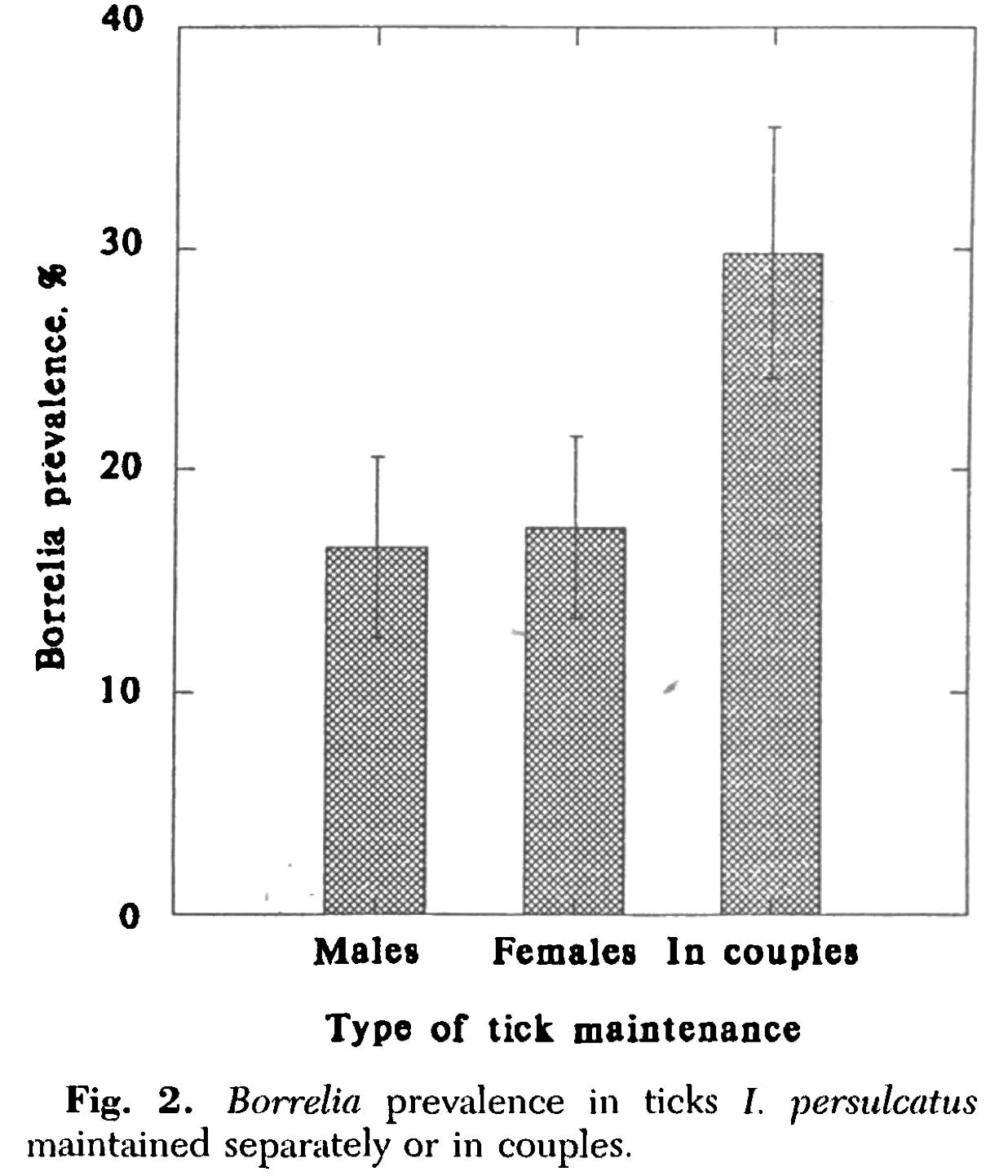
Then, in the 1990s, Russian researchers demonstrated a similar pattern in hard ticks—specifically Ixodes persulcatus, a close cousin of the deer tick (Ixodes scapularis). When they paired infected male ticks with uninfected females, the infection rate in females soared compared to those housed alone. In other words: mating triggered transmission.
Even more fascinating, the efficiency of this transmission depended on the Borrelia species involved. B. garinii (a Eurasian Lyme species) transferred readily from male to female ticks. B. afzelii, on the other hand, didn’t seem to transmit well unless the male tick was co-infected with B. garinii.
So what does this mean for humans? Not much. But also…not nothing.
These findings show that Borrelia can migrate into tick reproductive organs, and that it’s viable and infectious there. If male ticks can carry Borrelia in their gonads and pass it to females through seminal fluids, it raises the question: Might male mammals—or female mammals, for that matter—sometimes harbor Borrelia in genital fluids and transmit it the same way?
The tick data suggest that Borrelia doesn’t rely exclusively on blood-to-saliva transmission. It’s opportunistic. It adapts to its host. And given the right ecological context, it may exploit routes we’ve been too quick to dismiss.
Clues in Humans
Studying sexual transmission of Lyme disease in humans is fraught with challenges. Ethically and logistically, we can’t run experiments that expose people to Borrelia burgdorferi through intimate contact. That means we’re left with pieces of a puzzle that collectively suggest something may be missing from the mainstream narrative.
One of the first to raise this possibility publicly was dermatologist Gregory Bach. In 2001, he presented a curious observation: Lyme patients with infected sexual partners often struggled to fully clear the infection with antibiotics, almost as if they were being reinfected. He collected genital fluid samples—semen and vaginal swabs—from Lyme patients and their healthy partners. By culture and microscopy, he claimed to detect Borrelia spirochetes in about 40% of the healthy partners, many of whom had no tick bite history.
But this work never made it to publication. It was presented as a conference abstract, leaving major questions unanswered: Were the samples properly controlled? Was DNA testing used? Could the cultures have been contaminated?
Still, Bach’s report cracked open a door that others began to peer through.
In 2003, a more detailed hypothesis emerged from Houston, Texas—a city far from Lyme-endemic regions. Physicians documented a cluster of chronically ill patients who tested positive for B. burgdorferi by both antibody and PCR, despite having no known tick exposure. Perplexed, they proposed a dual-pathway model: ticks may seed initial infections, but secondary transmission between humans could sustain and spread the pathogen. Their mathematical modeling suggested that if even modest rates of human-to-human transmission occurred over centuries, prevalence could climb dramatically, perhaps even reaching 15% of the population.
Around the same time, Lyme specialist Raphael Stricker took a different approach: He studied couples in which only one partner had a tick bite history. Stricker found that the bitten partner typically had more severe disease and a lower CD57 cell count (a marker associated with chronic Lyme), while the unbitten partner often had milder symptoms. By contrast, couples in which both had known tick bites showed no such asymmetry. He interpreted this as potential evidence of intimate partner transmission: Perhaps the unbitten partner had acquired a smaller bacterial load or a different strain through sexual contact, leading to a subtler illness.
The most concrete evidence to date comes from a 2015 study by Middelveen and colleagues. They recruited 13 patients diagnosed with Lyme disease (including heterosexual couples) and healthy controls, and collected semen and vaginal swabs. The samples were cultured for up to four weeks in Borrelia-specific media, then analyzed using dark-field microscopy, silver staining, immunofluorescence, and PCR.
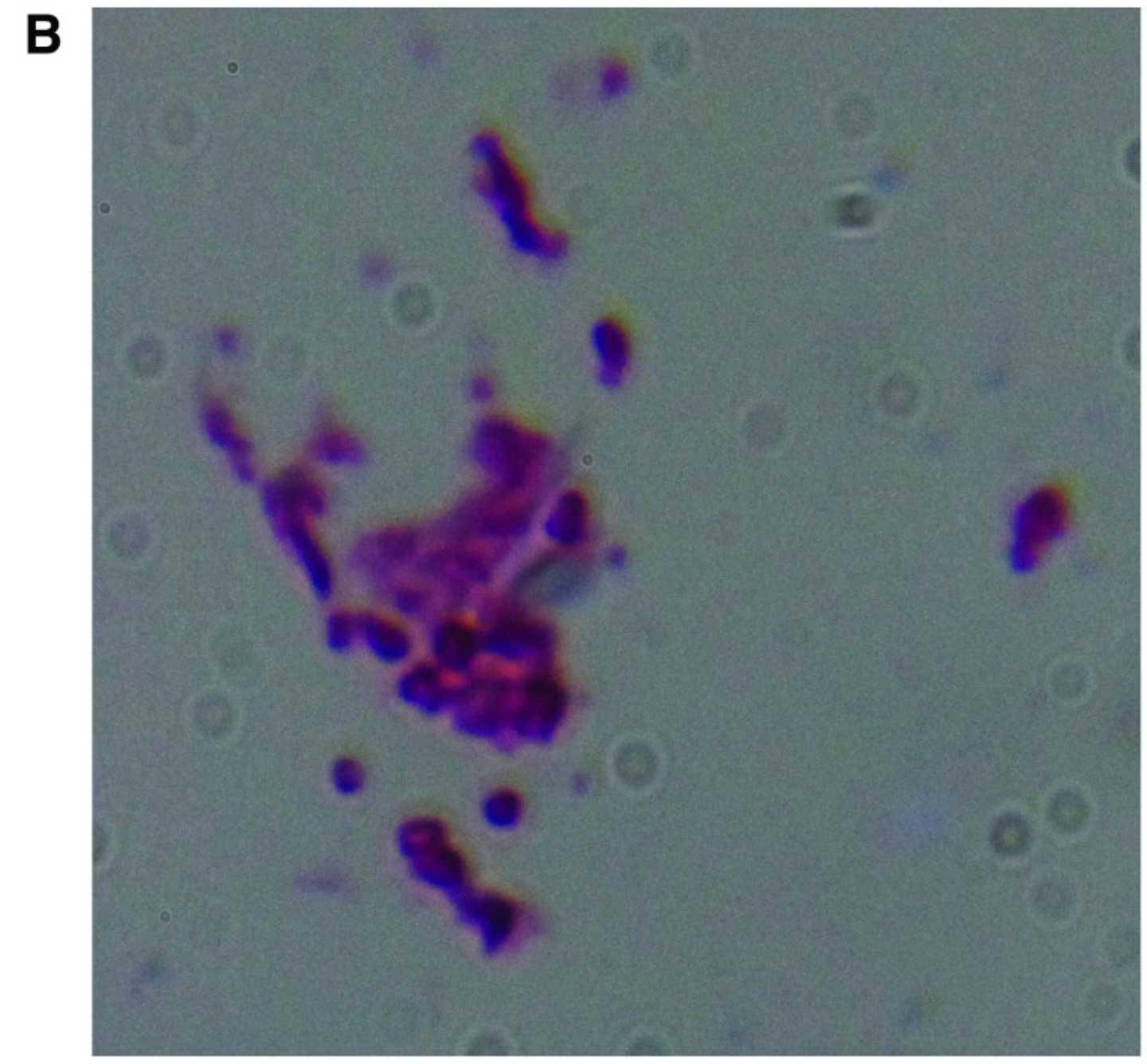
All but one of the patients had live, motile Borrelia visible in their cultured genital secretions. None of the healthy controls did, and species typing showed these were not syphilis bacteria, but B. burgdorferi and even B. hermsii in one case.
In three of the Lyme-affected couples, both partners harbored identical Borrelia strains in their genital fluids. In at least one of these cases, only one partner had a known tick exposure.
These results aren’t strong evidence of sexual transmission by any means. But they are hard to dismiss. If Borrelia can live and remain viable in semen and vaginal secretions, if it can be recovered and cultured from these fluids, and if couples share indistinguishable strains, then at minimum, the question deserves to be taken seriously.
An Epidemiological Puzzle
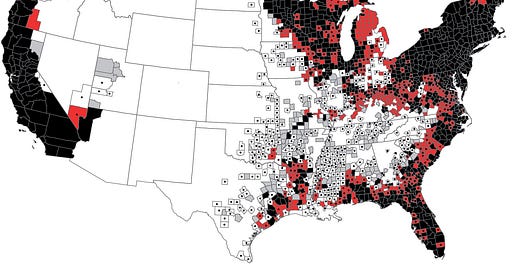
According to the CDC, Lyme disease infects around 476,000 people in the U.S. per year. But that number seems too high to be explained by tick bites alone.
Researchers Rudenko and Golovchenko walk through the math:
About 1.5 million tick bites are reported in the U.S. annually.
Only ~2% of tick bites lead to infection.
That yields just 30,000 new infections—barely a tenth of what’s reported.
And yet, cases are climbing. So where are the extra infections coming from?!
One possibility is that tick bites are simply underreported. Many people may not notice a bite, especially from tiny nymphal ticks, or may not connect a rash or flu-like symptoms to an insect encounter days or weeks prior.
But even accounting for that, the gap remains striking. Could we really be undercounting tick bites by a factor of 10 or 15? Perhaps. But that would mean millions of people are being bitten by ticks every year without knowing it. That hypothesis, too, demands explanation.
And it still can’t explain everything. Lyme cases are rising even in areas with low tick densities, and clusters often emerge in patients with no known outdoor exposure or recollection of tick contact. The geographic spread of Lyme into urban zones, southern states, and non-endemic countries often exceeds what local tick ecology can support.
Which brings us back to the core puzzle: if tick bites alone don’t account for the scale, the spread, or the patterns of disease, what else might be going on?
Maybe not all Lyme infections come from ticks. Maybe some come from humans transmitting to other humans.
The CDC forcefully rejects this position, however. As they put it, Lyme is
“strikingly different from the nationwide distribution of sexually transmitted diseases.”
Indeed, 96% of confirmed U.S. Lyme cases occur in a tight cluster of northeastern and upper midwestern states. This geographic concentration is often cited as strong evidence against sexual transmission, which, like syphilis or chlamydia, would be expected to show a more uniform distribution across the country.
But that logic may be misleading…
First, CDC data depend heavily on serological tests which, as we’ve seen, miss many cases. In this same vein, CDC-positive results aren’t consistently reported. In Colorado alone, our family saw three positive Western blots from three different labs, only one of which was formally reported to the state. The others, ordered via different routes, fell through the cracks.
Second, sampling bias is baked into the system. Clinicians outside Lyme “hot zones” are less likely to suspect it, less likely to test for it, and more likely to dismiss positive results. Even the labs themselves apply stricter interpretive criteria in non-endemic regions.
Third, Borrelia is a stealth pathogen. It can hide, shift shape, and evade detection in ways that confound standard diagnostics.
The 96% statistic may not tell us where Lyme is; it may just tell us where doctors are trained to look. So how do we explain the numbers?
One possibility is that primary infections begin with tick bites in endemic areas, but then spread slowly through secondary routes like sexual and congenital transmission. That model would explain the geographic clustering around tick habitats, while allowing for cryptic human-to-human spread outside those zones.
It’s also possible that sexual transmission of Lyme is simply less efficient than with other STIs. Maybe it requires a high bacterial load, or certain mucosal vulnerabilities, or immune suppression.
Intriguingly, as we saw in the tick studies, one Borrelia species (B. afzelii) only transmitted venereally when co-infected with another species (B. garinii), hinting that transmission efficiency may depend on the microbial context. Perhaps one strain boosts another’s infectivity or helps it breach new barriers. If similar dynamics occur in humans, it could help explain why person-to-person spread is sporadic, patchy, or goes unnoticed: It might require specific conditions, co-infections of specific strains, or host states that we don’t yet understand.
That’s speculation. But it’s not outlandish. And if the tick-only model were airtight, we wouldn’t need to entertain such speculation in the first place.
“No Credible Evidence”
If you visit the CDC’s webpage on Lyme disease transmission, you’ll find an unequivocal statement:
“There is no credible scientific evidence that Lyme disease is spread through touching, kissing, or sexual contact.”
But when you look at the citations backing it, it’s pretty flimsy. The CDC references just three sources: The two animal studies from the 1990s that failed to detect transmission between rodents (Moody, 1991; Woodrum, 1999), and a 2001 review that, oddly, doesn’t address sexual transmission at all. The CDC cites this paper to argue that “the biology of the Lyme disease spirochete is not compatible with this route of exposure.” But the review never evaluates that claim. Instead, it offers a comparative genomics perspective on B. burgdorferi and T. pallidum, noting differences in vector ecology and lipoprotein profiles, but doesn’t examine whether Borrelia can be transmitted via semen, vaginal secretions, or genital colonization. This isn’t a study that refutes sexual transmission, rather, it never asks that question, yet it is still being used as definitive evidence against it. That kind of citation logic should raise red flags.
There’s no mention of the multiple animal studies that found evidence of sexual transmission. No mention of the well-documented venereal spread of Borrelia between mating ticks. No mention of live spirochetes cultured from human semen and vaginal fluid. No mention of the identical Borrelia strains found in sexual partners. The silence is selective and conspicuous.
It’s worth noting that while the idea of sexual transmission is dismissed for lacking strong evidence, the tick-bite-only model of transmission is itself supported by surprisingly few human studies. This double standard raises the bar unevenly, requiring overwhelming evidence for new hypotheses while accepting older assumptions with far less scrutiny.
As a scientist, you want to believe that public health agencies are rigorous and impartial. But the CDC’s position hinges on selective citation and seemingly overconfident language.
Where Does This Leave Us?
Lyme disease has long been framed as a tick-borne infection confined to certain regions, with a clear mode of transmission and a clear course of treatment. But the story emerging from decades of research is far less clear.
We’re dealing with a pathogen that shape-shifts to avoid detection. We’ve seen evidence of congenital infection, a risk that’s increasingly being acknowledged among mainstream authorities. We’ve seen evidence of viable spirochetes in genital secretions. And we’ve seen patterns of spread, both geographically and interpersonally, that don’t align with the tick-bite model alone.
Having now read dozens of articles and skimmed dozens more, I’m still agnostic as to whether Lyme can be sexually transmitted. But my prior that it could be is much greater than it was before I started. And if even a small fraction of Lyme disease cases stem from sexual transmission, the implications are enormous. It would shift how we screen, how we counsel patients, and how we educate clinicians and the public. It would demand that we widen our diagnostic lens to include atypical presentations and non-endemic regions. It would force us to reconsider what kinds of surveillance and caution are warranted.
Science progresses by reconciling anomalies, and the science of Lyme transmission has given us plenty to work with.